Reprogramming the Tumor Immune Microenvironment for Cancer Therapy
Myeloid cells are the most abundant immune cells in multiple cancer types. They play key roles in cancer progression via diverse mechanisms, such as mediating tissue remodeling, driving local inflammation, suppressing or stimulating anti-tumor T cells. However, no effective strategy is currently available to target these cell types for cancer therapy. The overarching goal of our research is to develop an in-depth and broad understanding of transcriptional and epigenetic reprogramming of myeloid cells in the tumor microenvironment (TME). This will reveal novel regulatory mechanisms unique to tumor-associated myeloid cells that can serve as targets of precision cancer immunotherapies while preserving immune surveillance in healthy tissues.
Combining advanced genetically engineered mouse models, multi-omics, high-dimensional imaging and cutting edge tools in cellular immunology and genetics, we aim to decipher how distinct factors in the TME such as the microbiota, the peripheral nervous system, as well as the impact of the genetic makeup and immunogenicity of cancer cells that may differentially shape the myeloid cells.
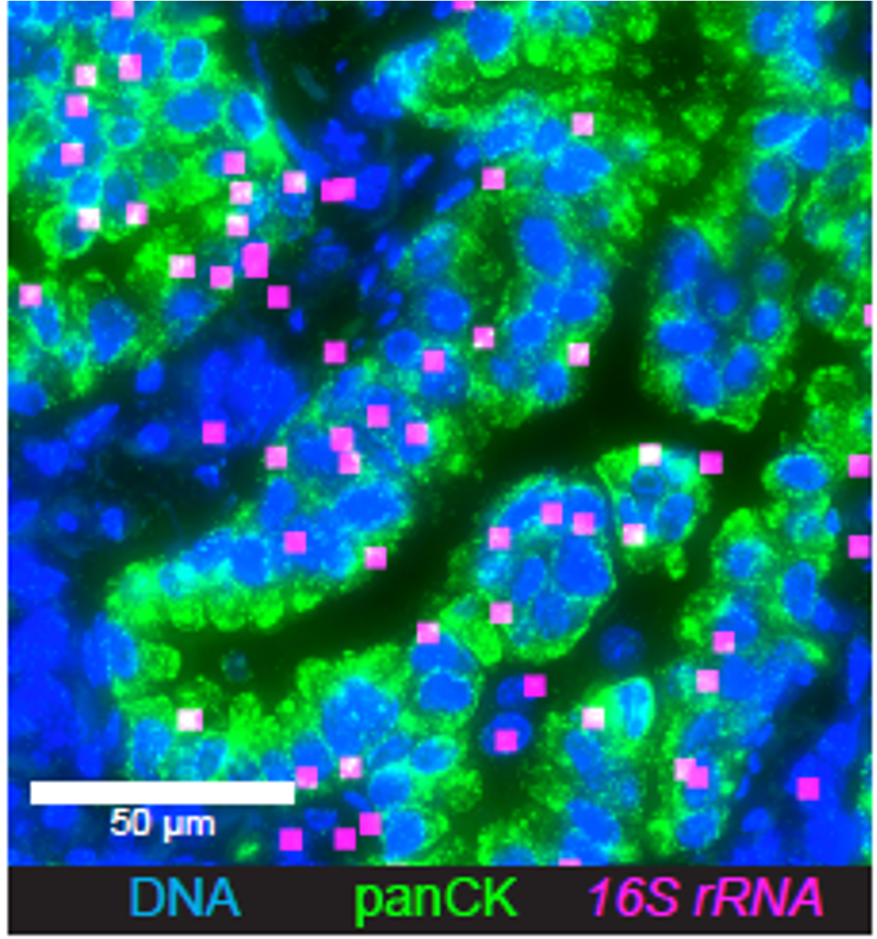
Tumor-associated microbiota
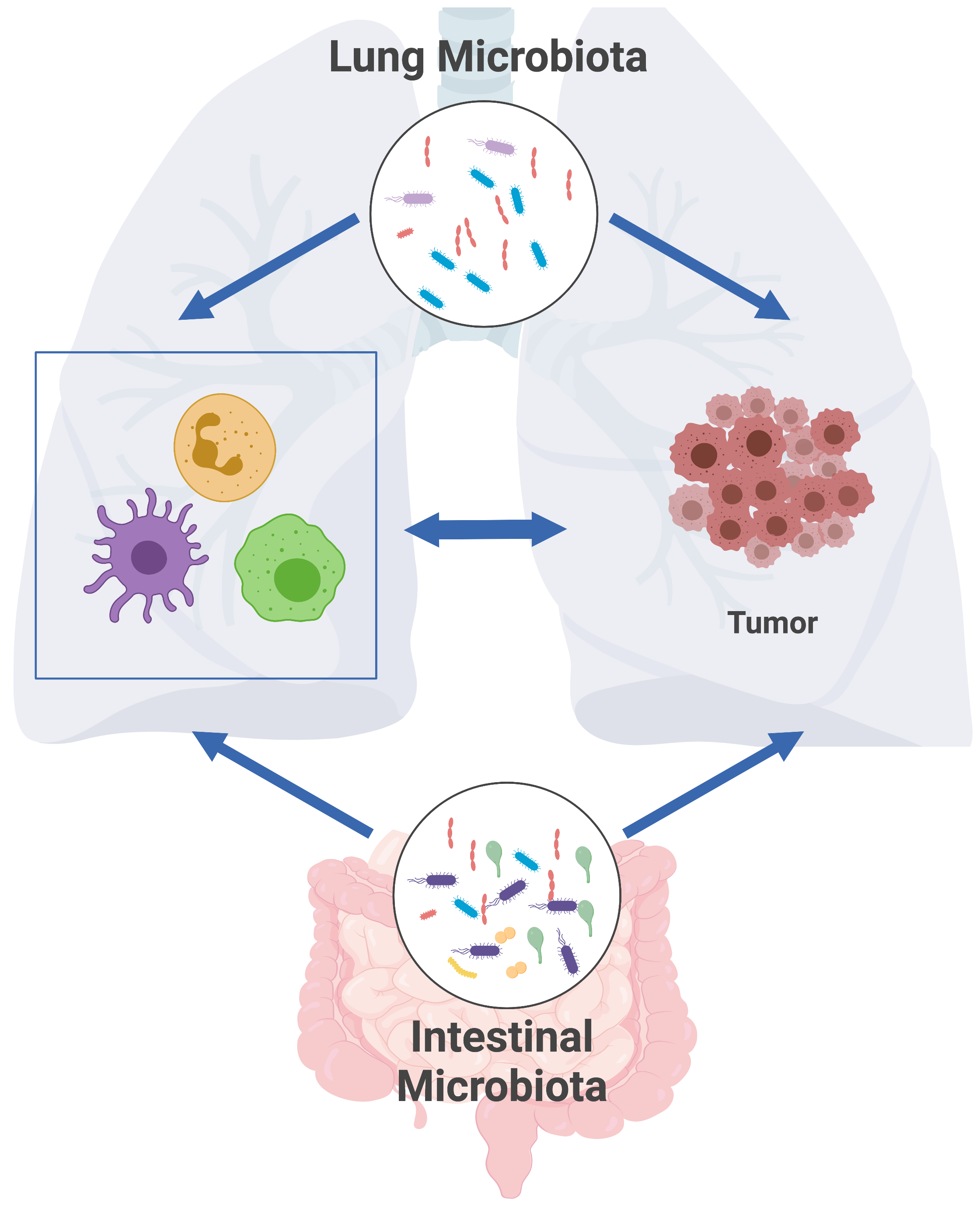
gut-lung axis
neuro-immune interaction
“cold” vs. “hot” tumors